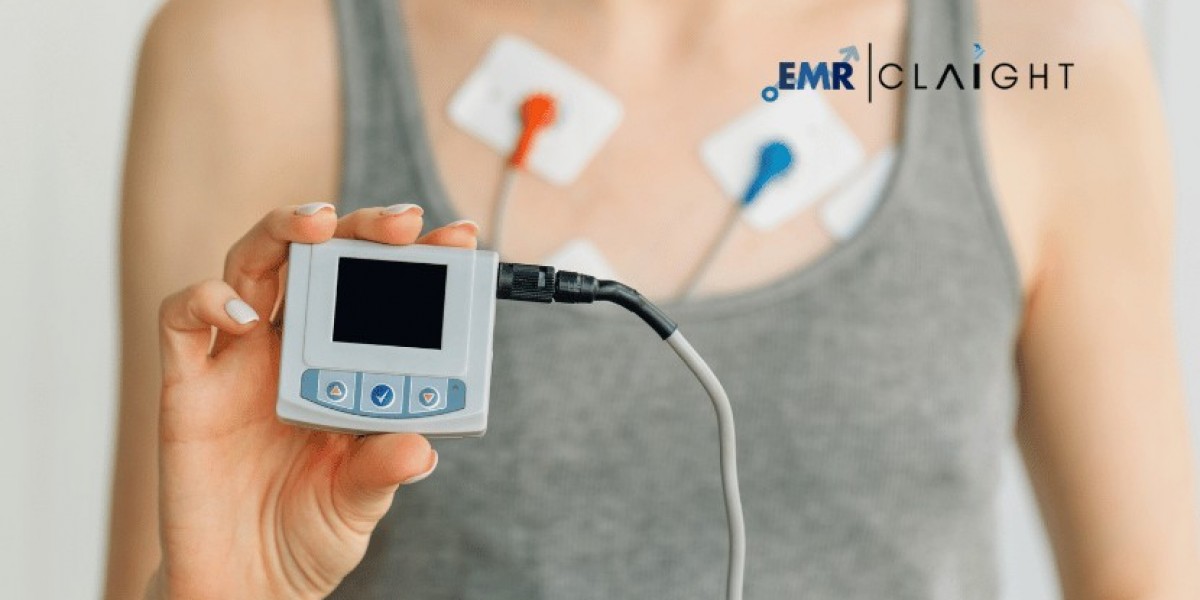
Event monitors are essential medical devices used to track and record irregular heart rhythms or events over extended periods. These portable devices are designed to help healthcare providers diagnose and monitor conditions such as arrhythmias and other cardiac irregularities. As cardiovascular diseases continue to rise globally, the demand for event monitors has increased significantly. Establishing a manufacturing plant for event monitors requires advanced production techniques, stringent quality control measures, and compliance with medical device regulations to meet market demands effectively.
Overview of Event Monitors
Event monitors are wearable or portable medical devices that record electrical signals of the heart when triggered manually or automatically during irregular heart activity. These devices are lightweight, user-friendly, and designed for prolonged monitoring, offering valuable diagnostic insights for both patients and clinicians. Modern event monitors often incorporate wireless technology, real-time data transmission, and cloud-based storage for seamless reporting and analysis.
Get a Free Sample Report with Table of Contents@ https://www.expertmarketresearch.com/prefeasibility-reports/event-monitor-manufacturing-plant-project-report/requestsample
Key Considerations for Setting Up the Manufacturing Plant
Setting up an event monitor manufacturing plant requires strategic planning and execution, focusing on location, raw materials, production processes, and compliance with industry standards.
1. Location and Site Selection
The location of the plant is critical to operational efficiency. Key factors include:
- Proximity to suppliers of electronic components, sensors, and casings.
- Availability of skilled labour for assembling and testing devices.
- Accessibility to utilities like electricity and waste management systems.
Adequate space for production facilities, testing labs, and administrative operations is also essential.
2. Raw Materials and Procurement
High-quality components are essential for producing reliable event monitors. These include:
- Electronic Components: Microprocessors, circuit boards, and memory units.
- Sensors: ECG electrodes and biosensors for accurate heart monitoring.
- Power Sources: Rechargeable batteries or power modules for portability.
- Casing Materials: Lightweight and durable materials for the device's outer shell.
- Software Systems: Pre-installed diagnostic algorithms and data storage platforms.
Reliable supplier partnerships ensure consistent availability and quality of raw materials.
3. Manufacturing Process
The production of event monitors involves several precise steps:
- Design and Prototyping: Developing functional and ergonomic designs for the device.
- Component Assembly: Integrating sensors, circuit boards, and processors into the device casing.
- Software Installation: Embedding diagnostic software for data recording and analysis.
- Calibration and Testing: Ensuring the device meets accuracy and performance standards.
- Packaging: Sealing devices in tamper-proof packaging with user manuals and accessories.
4. Quality Control
Stringent quality control measures are critical for manufacturing reliable medical devices. Testing parameters include:
- Accuracy of signal detection and recording.
- Device durability and performance under varying conditions.
- Compliance with safety standards for medical-grade electronics.
Regular testing and certification ensure compliance with industry regulations and enhance consumer trust.
5. Regulatory Compliance
Adherence to medical device regulations and industry standards is mandatory. This includes:
- Proper labelling with instructions, certifications, and safety guidelines.
- Certifications for ISO, FDA, or CE, depending on the target market.
- Compliance with electronic waste disposal and environmental regulations.
Equipment and Technology
Setting up an event monitor manufacturing plant requires specialised equipment to ensure precision and efficiency. Key equipment includes:
- Surface Mount Technology (SMT) Machines: For assembling electronic components.
- Sensor Calibration Tools: To ensure accuracy and sensitivity of ECG electrodes.
- Software Testing Systems: For embedding and validating diagnostic algorithms.
- Packaging and Labelling Machines: For secure and compliant product packaging.
- Quality Assurance Tools: For testing device functionality and durability.
Automation technologies can streamline production, improve efficiency, and maintain consistent quality.
Workforce and Training
A skilled workforce is essential for the successful operation of the plant. Key roles include design engineers, assembly technicians, and quality assurance professionals. Regular training programs keep employees updated on manufacturing techniques, safety protocols, and regulatory compliance.
Packaging and Distribution
Proper packaging is crucial to maintain the quality and usability of event monitors. Packaging options include sturdy boxes with protective inserts to prevent damage during transit. Efficient logistics networks ensure timely delivery to hospitals, clinics, and medical device distributors.
Environmental and Safety Aspects
Sustainability and safety are integral to the production process. Measures include:
- Waste Management: Recycling electronic components and minimising production waste.
- Energy Efficiency: Using energy-efficient machinery to reduce the plant’s carbon footprint.
- Worker Safety: Providing protective gear and regular safety training to minimise risks.
Market Applications and Trends
Event monitors cater to a wide range of medical applications and consumer markets:
- Cardiology Clinics: For diagnosing and monitoring heart rhythm disorders.
- Home Healthcare: Offering portable solutions for remote monitoring.
- Telemedicine Services: Integrated with cloud platforms for real-time reporting.
- Sports Medicine: Used for monitoring heart health during physical activities.
Emerging trends include:
- Wearable Technology: Compact, discreet, and comfortable designs for long-term use.
- AI Integration: Enhanced diagnostic capabilities with real-time data analysis.
- Sustainability: Use of eco-friendly materials and recycling initiatives.
Challenges in Manufacturing
Manufacturers face challenges such as maintaining consistent quality, managing high production costs, and meeting stringent regulatory requirements. Addressing these challenges involves:
- Investing in advanced production technologies and quality control systems.
- Building strong relationships with suppliers for reliable sourcing of components.
- Innovating product designs to meet consumer demand for compact and user-friendly devices.
By focusing on these critical aspects, businesses can establish a successful event monitor manufacturing plant and cater to the growing global demand for advanced cardiac monitoring solutions.